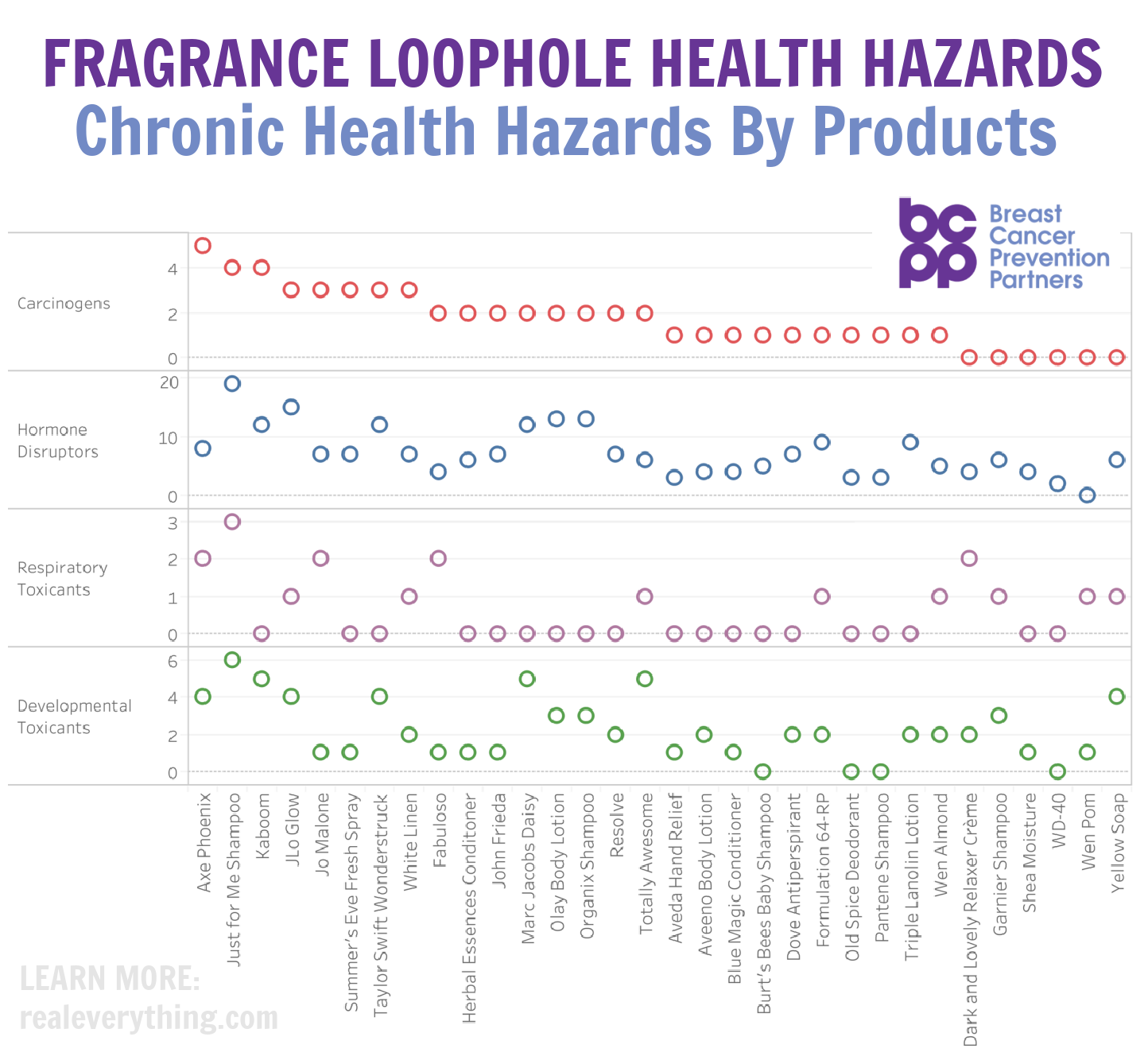
The thing about science is that in and of itself, by definition, it should be constantly changing. By the very nature of constant learning, we discover things we didn’t fully understand before. And where sometimes that leads to groundbreaking bans of dangerous ingredients, sometimes we also have to be willing to open our mind to the idea that we were wrong, and things are safer than they originally seemed. Now don’t get me wrong, I’ll always err on the side of “avoid until proven safe” versus the States’ “let it be used by consumers until proven unsafe” philosophy. But in today’s case, here are 5 Personal Care Ingredients I Use (that alarmists warn you about):
- Water
- Retinyl Palmitate
- Phenoxyethenol
- Synthetic Fragrance
- Butyloctyl Salicylate SPF Boosters
As with all things in life, there is nuance and complexity – so let’s get into it!
Water
Why is water bad in personal care? It’s not. Good gravy if I have to hear more about the wonders of “waterless beauty” I’m going to firehose the person telling me about it. For years this has been the most ridiculous “warning” I’ve heard in this space. Let’s de-bunk this once and for all:
Waterless Beauty
First, we need water. “Water is absolutely essential for the normal functioning of the skin and especially its outer layer, the stratum corneum (SC).” (1) Our bodies are at least half water, 75% – 55%, reducing as we age. Water is part of cellular health; it is “essential for cellular homeostasis and life.” (2) And, our bodies also need fats.
We must have both for healthy skin.
One of our body’s natural resources to use that moisture for skin health is Hyaluronic Acid. It is a natural substance found in the fluids of eyes and joints. Various forms of hyaluronic acid are used for beauty, both skincare and cosmetic procedures. Without water, hyaluronic acid in a product cannot work effectively. And there are many water-soluble vitamins used in skincare that need it to carry the nutrients into your skin for proper absorption.
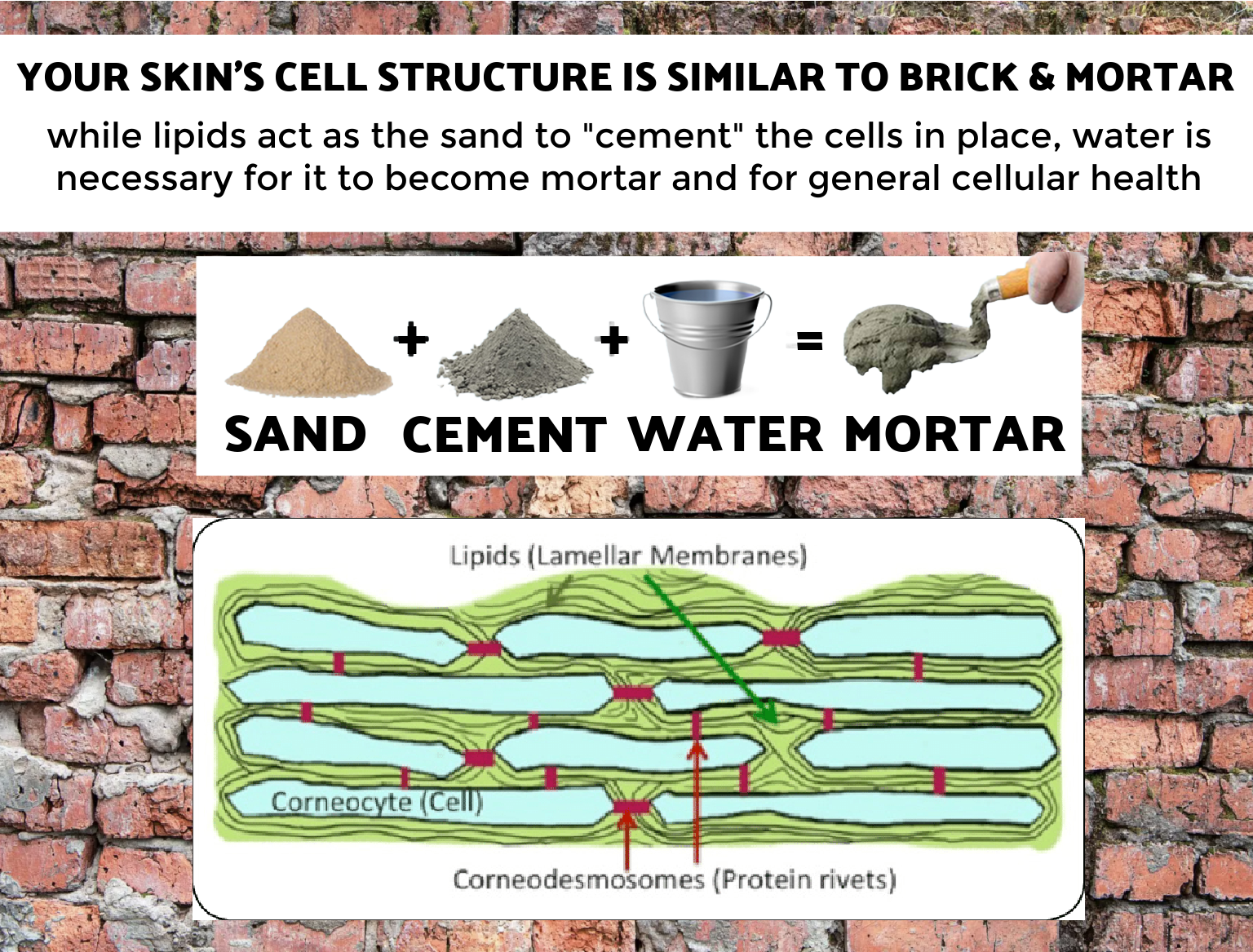
While I have often discussed the importance of lipids for our skin health, they cannot act alone. Your skin cell’s structure is similar to brick and mortar. While lipids act as the sand to “cement” the cells in place, water is necessary for it to become mortar. And for general cellular health!
Reverse Osmosis Water
The other heckle I’ve witnessed regarding water in beauty products, is that tap water contains contaminants. It could, yes. But just because a label doesn’t specify “reverse osmosis water”, it doesn’t mean they’re not doing so. For example, Beautycounter used the most advanced RO system to filter water possible, but the product label simply said, “water”.
Why is reverse osmosis water better in skincare? From the simple standpoint of: the purer the water, the purer the skin. You’ve probably heard that “hard water” can affect your hair quality. And it can also affect your skin. Hard water simply means water with more sediment, more minerals, or potentially contaminants.
Filters help remove things that could potentially have a detrimental effect on hair and skin, like chlorine and heavy metals. (side note: we love this affordable shower filter). The cleaner, softer water is better absorbed by the skin. And RO even more, as they make water molecules smaller, which can penetrate and hydrate the skin better.
So before you see water in the ingredient list and think “oh, they’re just watering down my product.” Think twice. Not only could that be a necessary component to carry the water soluble vitamins and hyaluronic acid into your skin, but it’s also likely been filtered even if it doesn’t state as much. Though, it’s always good to check!
Learn more:
Retinyl Palmitate (Vitamin A)
Before we can talk about Retinyl Palmitate, we need to understand how our body uses Vitamin A, which is the source of several retinoids that I’ll explain the difference between.
Vitamin A plays a crucial role in regulating various physiological processes of the body and maintaining vision and immune system function to support skin health and cell growth. Although vitamin A is essential for the body, excessive intake can pose various adverse effects, disrupting the body’s equilibrium and overall well-being. Vitamin A toxicity, also known as hypervitaminosis A, can result from either the excessive consumption of vitamin A and related compounds or its topical application. (3)
Why would we want to potentially increase Vitamin A if there is potential for harm?
When converted in our bodies to retinoic acid, it slows down collagen breakdown, stimulates rapid cell turnover, and collagen and elastin formation, thereby minimizing the appearance of fine lines and wrinkles. They can also prevent acne bouts, as well as reduce dark spots due to acne or other scarring.
As we consider your overall tolerance, remember that you are often consuming natural sources of Vitamin A. It is found in: breakfast cereals, juices, dairy products, fruits and vegetables (leafy green vegetables, orange and yellow vegetables, tomatoes, red bell pepper, cantaloupe, mango), beef liver, fish oils, eggs, and fortified foods.
When used in skincare, all forms ultimately convert to retinoic acid, which is what provides skin-changing results. Because each step is needed for conversion weakens the action of that resulting derivative, the more conversions that are needed the least intense and more gentle on the skin.
The highest absorption would be in the following order:
- Retinoic acid and Isotretinoin are most potent Retinoids, are the most intense as they do not need any conversion by our body, example: Tretinoin a prescription-strength version which requires doctor oversight
- Retinal/Retinaldehyde and Hydroxypinacolone Retinoate (Granactive) – these are converted to retinoic acid in a one-step process
- Retinol, the most commonly used in skincare, converts to active retinoic acid in two steps (retinaldehyde then retinoic acid)
- Retinyl Esters, such as Retinyl Palmetate and Retinol Propionate are converted in three steps, making it the mildest form of retinoids, which can be used by those with sensitive skin. (4)
- Beta Carotene, the red-orange carotenoid antioxidant pigment in fruits and vegetables, was determined to be a topical precursor of epidermal vitamin A. (8)
That last study blew my mind and really helped me understand how determination of safety is really based on 1) dosage, and 2) usage.
But first let’s dig into the updated science on it all.
Updating the Science
In the 20th and early 21st centuries, studies were done with mice that led science to believe that all forms of topical retinoids were potentially harmful to human health. (5) Which is why the EWG noted it as a red 9. But EWG has since changed their stance, even verifying products with Retinyl Palmetate. Why?
Recent research (2017, 6) has found flaws in those studies and given more nuance to topical use of retinoids.
The Panel thoroughly reviewed a 2012 NTP photococarcinogenicity* study on retinyl palmitate and retinoic acid, including an expert panel’s review of the study findings. The Panel noted the methodological flaws and, on that basis, determined that the findings could not be properly interpreted to suggest additional risks associated with these ingredients. A second NTP photococarcinogenesis study to address flaws in the original study may be considered when the new study is completed.
New toxicity data on retinol and retinyl palmitate and data on retinoic acid, retinyl acetate, and retinyl propionate that became available since the final safety assessment was issued were reviewed. The Panel recommended that data on residual levels of retinyl palmitate and retinol that remain in the epidermis following ingredient application in the presence of UV light be included when the second NTP study data are reviewed and that retinoic acid be removed from the report because it is a US Food and Drug Administration–approved drug.
What does that mean?
In summary, in review of those earlier studies it was found that results cannot be used due to flaws in the experiments themselves. And, in reviewing new data since, it was determined that potential for toxicity is not an issue and should be updated because it is now approved by the FDA. As it relates to photocarinogenicity, a follow-up study is recommended to determine if there is increased risk when retinoids topically stay on the skin with UV exposure. *photocarcinogenicity is a fancy term for increased risk of skin cancer due to UV exposure
To further validate these findings, here is another double-blind controlled study demonstrating retinoids do not increase inflammation. Thus invalidating that the claims that low levels of topical retinoids would be a burdon on the liver.
Unexpectedly, this study found no significant changes in cytokine levels after six months of RP supplementation in MS patients.
And then the study that blew my mind: beta-carotene applied topically is converted into retinyl esters (same thing as Retinyl Palmitate) by human and mouse epidermis and thus is a precursor of epidermal vitamin A. There are a lot of products I’ve used without concern for safety that contain beta-carotene, such as the turmeric in the beloved Beautycounter All Bright C Serum.
My Personal Approach is Nuanced, just like the findings
I had previously been avoiding all retinoids for… ever. Now, with updated science and recognition of that by EWG, I have begun using several products with low levels of Retinyl Palmitate (and can legitimately see a difference). The specific products I am using are in a cleanser (so it is washed off) and creams I am using at night. I no longer have any pause to use or recommend.
I will not be using or recommending any product that would stay on the skin during the day. Science is still inconclusive on if photocarcinogenicity is increased, and I will err on the side of safety. It is critical to use SPF daily regardless of when you use any retinoid!
Learn more:
Phenoxyethanol
There is a backlash against pheoxyethanol in the clean beauty space, but that is coming from natural brands and genuinely concerns me. If a company is not using a preservative of some kind, we find cases of bacteria growing in skincare that can be truly harmful to human health. (9) As I mentioned earlier, many skincare products have water in them. Preservatives ensure your serum doesn’t turn into a breeding ground for mold.

And, most of the brands using this talking point aren’t testing their products for safety. Especially in all-natural mineral-based products, each batch needs to be tested for heavy metals and other contaminants like asbestos, commonly found in nature. It’s a great marketing ploy, but when we point a finger at someone else we’ve got three pointed right back at us.
But what about the safety of Phenoxyethanol?
Pheoxyethanol is a preservative. It is a favorite among many clean beauty brands because it is one of the safest preservatives on the market. Comparatively in many main market products, preservatives would include parabens and formaldehyde releasing ingredients. When looking up preservatives in the EWG Skin Deep database, you will find they rank phenoxyethanol in a range of 2-4 on a scale of 10 potential health risks.
EWG has determined that phenoxyethanol can be used safely and is allowed in it’s Verified program. EWG takes a strong stance to err on the side of caution. And still, has reviewed the science and deem phenoxyethanol safe. Scientific studies and findings are at the core of considering, both to EWG’s safety rating as well as what I choose to use in my personal care products (and beyond). If we look at the plethora of science showing the health risks associated with parabens and formaldahyde, these cannot be replicated in any sort of similar why with phenoxyethanol.
Learn more:
Synthetic Fragrance (and colors)
Once a time not-too-long-ago, the clean beauty world used the phrase “synthetic fragrance-free.” And then we learned better.
Turns out only natural fragrance ingredients can not only contain endocrine disruptors altering hormone health, but they are often bad for the ecological reasons as well. Safer synthetic ingredients allow for customized solutions that can ensure avoidance of known harmful additives (like phthalates, often added to make scents “stick”) and tested for sensitive folk.
- Not everything in nature is safe (asbestos, heavy metals, poison ivy and snake venom, I’m looking at you).
- Not everything man made is unsafe (this is where full transparency in ingredients and testing for safety matters).
For those that value Earth’s precious resources, safer synthetic offers a way to create more sustainable scents. Just because something says “natural” or “only fruit oils in the perfume” doesn’t mean anything, since our laws allow for hidden chemicals without disclosure – many “natural” scents contain phthalates and other potentially harmful additives.
With new information, I now no longer fear synthetic fragrance. I do, however, ensure that not only are all ingredients disclosed, but that they are testing for contaminants as well. The three brands I choose to use and partner with that have scents I have validated are:
Learn more:

Butyloctyl Salicylate SPF Boosters
Honestly, I hadn’t even heard of this ingredient as potential concern until some of you asked me about it. Lab Muffin brought it up, and I want to summarize my take, as well as things to consider as consumers. Here’s her opening:
There’s a sort of industry secret with mineral sunscreens: a lot of them contain unregulated chemical sunscreens. Which isn’t always a big problem… but it also kind of is. (11)
So, um… all personal care products labeled with SPF are regulated in the States:
Sunscreens have been regulated by FDA since the 1970s, but they have garnered a lot of attention recently. (12)
I’m here for a good scientific nerd-out. You put up images of chemical structures and immediately peak my interest. The problem, however, is that what is being explained does not align with the assumptions the author is making – which, if it weren’t painfully obvious, is not scientific.
Why isn’t butyloctyl salicylate classified as a proper sunscreen active ingredient, in that top box with the percentage shown? Well, it seems like… the manufacturer just decided not to go through the proper legal process.
So the argument here isn’t that the Butyloctyl Salicylate is harmful (as shown above, depending on usage it can be an EWG 1 and does show as an ingredient in EWG Verified products). But rather, that it’s not listed as an “active ingredient.”
Active vs. Inactive Ingredient
Rather than assuming, let’s look at FDA’s Regulation on the Labeling and Effectiveness Testing: Sunscreen Drug Products for Over-the-Counter Human Use Section IV (13) and the accompanying CFR (14), it actually very clearly defines what a manufacturer identify as Active ingredients so that we don’t need to guess why a company would label something Active vs. Inactive:
Active ingredient means any component that is intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease, or to affect the structure or any function of the body of humans. The term includes those components that may undergo chemical change in the manufacture of the drug product and be present in the drug product in a modified form intended to furnish the specified activity or effect.
Inactive ingredient means any component other than an active ingredient… We are not requiring specific details about what each ingredient does in the product. (14)
Why I’m Okay Using It
By definition, a “booster” would be indirect. And, Butyloctyl Salicylate is an emolient. So I can only imagine that this article and accompanying video are inentionally click-baity to grab attention and poke at clean brand’s “holes” (as is the case in other articles). I’ll leave you with her own words as to why I don’t worry about this ingredient at all.
Using butyloctyl salicylate is really widespread and accepted in industry – tons of brands have it in their sunscreens, from multinational brands down to small brands who probably don’t realize it’s in there. I think that’s because butyloctyl salicylate is probably quite safe most of the time. (11)
For reference, the Countersun sunscreen my very fair family uses does not contain Butyloctyl Salicylate. But, the Countersun Mineral Sunscreen Spray mist version does. This logically makes sense to me that it would need a “booster” as both an emollient and to assist in “sticking” on the skin, given the different application than a lotion or stick rubbed directly in. I have no concerns with my children using this mist, as I know it is tested for contaminants and uses non-nano zinc oxide (less harmful if inhaled).
Have other ingredient questions?
I love researching and helping people!
Want more info on our Real Life?
Never want to miss a post, sale, or deal? Join my Healthy Inside & Out e-mail list for more info on non-toxic living and safer skincare!
Recipes, parenting tips, and general lifestyle stuff goes out in our Real Everything newsletter, join here.
Sources
- Skin hydration: a review on its molecular mechanisms, PMID: 17524122
- Water, Hydration and Health, PMID: 20646222
- Vitamin A Toxicity, NIH’s National Library of Medicine publication, 2024
- The different types of retinol, explained by an expert, Dr Agarwal for Vogue India
- Topical beta-carotene is converted to retinyl esters in human skin ex vivo and mouse skin in vivo, PMID: 15335356
- Retinol and Retinyl Palmitate, Sage Journals Volume 36 Issue 5
- The effect of retinyl-palmitate on the level of pro and anti-inflammatory cytokines in multiple sclerosis patients: A randomized double blind clinical trial, PMID: 30640138
- Topical beta-carotene is converted to retinyl esters in human skin ex vivo and mouse skin in vivo, PMID: 15335356
- Herbivore’s moldy face-cream recall at Sephora underscores an ugly issue for natural beauty, Fast Company 2019
- No, Phenoxyethnol is not the new Paraben by Linday Dahl
- SPF Boosters: hidden Chemical Sunscreens in “Mineral Sunscreens” by Lab Muffin Beauty Science
- An Update on Sunscreen Requirements: The Deemed Final Order and the Proposed Order from FDA.gov
- Regulation on the Labeling and Effectiveness Testing: Sunscreen Drug Products for Over-the-Counter Human Use from FDA.gov
- CFR – Code of Federal Regulations Title 21 from FDA.gov